Seed Funded Projects - Chemical Engineering and Biotechnology
Professor Alex Routh, 2024
Medical diagnostics from drying of blood droplets
When blood drops are dried a number of transitions occur. As shown in Figure 1 the droplet is initially homogeneous. After about 8 minutes, red blood cells accumulate at the edge and consolidate, forming a solid. This region of solidified particles grows from the edge towards the centre of the droplet, as seen in the images up to 24 minutes. For dispersions comprising polystyrene particles the front of consolidated particles is seen to progress all the way to the droplet centre, but in the case of blood it is observed to stop at a specified distance. The two disparate regions (central and consolidated edge) then continue to dry as separate entities.
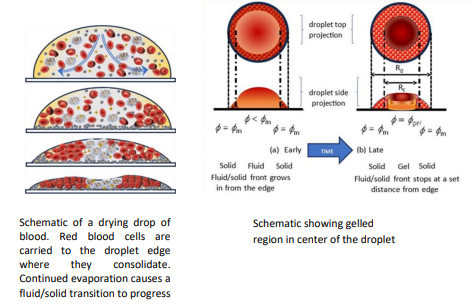
Figure 1: A droplet of human blood drying on a glass slide. The droplet diameter is approximately 4 mm. Image courtesy of Michael Hertaeg and reproduced from Phil. Trans. R. Soc. A 379:20200391.
We seek to relate the final dried morphology to the initial state of the blood. Doing this allows diagnostic decisions to be taken and the specific physiological conditions we are aiming to investigate are
Anaemia: The initial volume fraction of red blood cells affects the final morphology. By correlating these we are hopefully able to measure the initial hematocrit level and hence diagnose anaemia
Red Blood Cell rigidity: a number of conditions including malaria, sickle cell anaemia and thalassemia affect the rigidity of the red blood cells. We hypothesise that this affects the resulting crack pattern. By quantifying the dried pattern, we seek correlations with different conditions.
We have designed and built a droplet light transmission device. This allows the drying droplet to be followed in situ and the final droplet shape to be measured. The work has recently been published (Sheila J. Bhatt and Alexander F. Routh, Optical transmission profilometry for tracking time-resolved particle redistribution in sessile colloidal suspensions, Scientific Reports 14:637 2024).
Professor Róisín Owens, 2024
Integration of lung organoids into 3D bioelectronic devices for drug discovery
First, scaffolds were fabricated using a composite of conducting polymer and extracellular matrix protein. The following steps were then optimised for biomimetic tissue formation: fibroblasts were seeded into the bulk of the scaffold, filling the pores, and then a single-cell suspension of airway cells were seeded on top, with the aim of achieving a confluent monolayer of airway cells.
Unfortunately, shortly after submitting the progress report we faced large challenges with contamination issues in the stem cell institute tissue culture room, which unfortunately resulted in the loss of all of the organoid lines. The human organoid cultures were halted until the contamination was resolved. While the contamination issue was being resolved, we reverted to using human airway cell lines together with the fibroblasts.
In the meantime, Dr Joo-Hyeon Lee accepted a position at Sloane Kettering in the US, meaning that without a partner for the organoids, future work on lung would be challenging. We therefore reverted to working on gut models and have established another partnership at the Cambridge Stem Cell Institute, with Prof. Matthias Zilbauer, integrating gut organoids into our devices.
Dr Graham Christie & Dr Somenath Bakshi, 2023
Spore forming bacteria as cellular factories for lignocellulose processing
Enzymatic conversion of lignocellulosic crop-based waste materials to more useful carbohydrate monomers is challenging owing to the recalcitrant nature of the substrate. Current industrial practices in this regard are considered sub-optimal from environmental and circular economy perspectives. In nature lignocellulose is degraded by various soil fungi and bacteria. The catalytic properties of enzymes utilised by these microbes can be harnessed from a biotechnological perspective using recombinant versions of purified cocktails of enzymes, however the approach suffers from significant drawbacks in terms of expense and protein instability.
This project sought to address some of these problems by using synthetic biology approaches to engineer Bacillus subtilis spores that ‘displayed’ various lignocellulosic enzymes on or within the vicinity of the surface of the spore. The rationale behind this is that by incorporating lignocellulosic enzymes within the proteinaceous outer shell of the inherently robust bacterial spore, enhanced stability is conferred to the heterologous expressed enzymes. Outputs from the work indicate that spores of several novel strains expressed and displayed functional cellulosic enzymes, while ease of production, purification and re-cycling of the spore immobilised enzymes was demonstrated. Ultimately we anticipate that these strains will serve as a useful resource for future work in this area.
Dr Sam Stranks, 2021
Development of Novel X-Ray Scintillators for Medical Imaging
The ultimate goal of this activity is to develop efficient and stable perovskite materials for sensitive X-ray detection that outperform current technologies in medical imaging, security and food safety. The seed funding project was a resounding success, with the £10k already converting into over £1M in funding that is driving both academic and commercialisation efforts, as well as technical demonstration of radioluminescence from our films.
The activities contributed to an EPSRC award within the Transformative Healthcare Technologies scheme (EP/W004445/1) entitled ‘Revolutionizing Medical Imaging (ReImagine) through Ubiquitous, Low-Dose, Automated Computed Tomography Diagnostic Systems’ where we aim to realise new paradigms for X-ray imaging with a cross-school team of Dr Sam Stranks (CEB), Prof. Evis Sala (Radiology), Prof. Carola-Bibiane Schönlieb (DAMTP), Dr Fairen-Jimenez (CEB) and partners at Loughborough, Leicester and Leiden Universities, Cheyney, Scintacor, Immaterial, and GE Research.
Further funding was secured through the 2020 EPSRC Core Equipment Call to purchase a time-resolved radioluminescence X-ray system for advanced characterisation of our scintillators. This setup will come online in October 2021 as a Strategic Research Facility (SRF), future proofing the activity and providing unique equipment that will be useful for a number of researchers across the University and wider industrial community. Finally, the project has also boosted commercialisation efforts in this space through the award of an ERC Proof on Concept grant (Perovskite Scintillators for X-Ray Imaging) and an EPSRC IAA grant.
Professor Geoff Moggridge, 2021
Contact killing of coronavirus by copper impregnated polymers
This School Seed Fund Project successfully established that:
- Their best performing proprietary copper polymer technology kills >99.9% Mouse Hepatitis Virus (MHV) in under an hour, >100 times superior to Copper3D polymer (main competitor) via MHV splash testing on copper impregnated films with different copper concentrations.
- Impregnation and coating of polycarbonate samples resulted in a surface layer suitable for face mask applications with insignificant copper leaching of the samples demonstrated (much lower than drinking water limits).
- Nonwoven polypropylene filters for face mask application could be impregnated and coated, with breathability well within standard limits, with whole mask prototypes successfully coated by dip coating and spraying.
The project has led to a successful Wellcome Trust Enabling Technologies application, with the potential to also win Innovate UK funding.
Further to this SARS-CoV-2 splash tests on proprietary copper impregnated polymers films demonstrated >99% virus killing within an hour, 8 times faster than the competitor polymer.

myMaskFit has been working with Cambridge to apply the coating to their prototype mask shown, transparent masks are in production with the aim to eliminate the disposable FFP3 masks shortly, and with the custom fitting of these masks make them reusable saving waste, improving comfort and providing 99.9% filtration efficiency to our health workers
